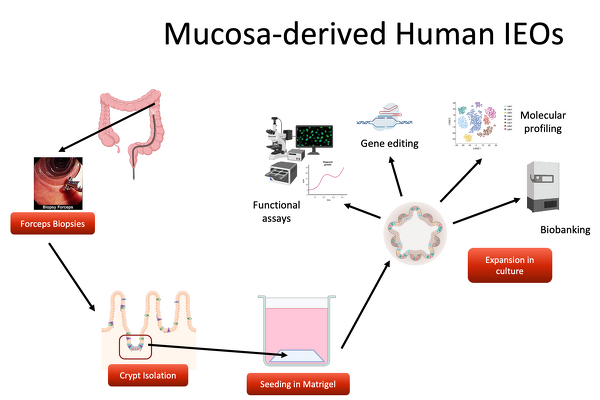
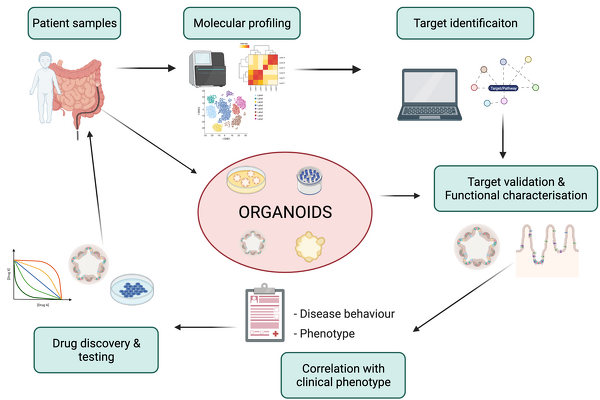
As one of the main experimental models, we use human mucosa derived intestinal epithelial organoids. Over the past 8 years, we have generated a large living biobank containing >1000 frozen organoid lines that form the basis for future translational research efforts. In addition to organoids derived from intestinal mucosal samples, induced pluripotent stem cells (iPSCs) can be differentiated into intestinal epithelium.
Current projects focus on the development of clinical biomarkers to guide treatment of children diagnosed with Inflammatory Bowel Diseases, test novel treatments targeting the intestinal epithelium using our organoid model and further develop 2D and 3D culture systems which allow complex co-culture of the intestinal epithelium with other cell types (e.g. immune and stromal cells) as well as the intestinal microbiota.